In today’s technology landscape, companies are continually making improvements to electronic devices. Bigger screens, better cameras, and smarter systems are just some of the improvements these companies promise to consumers with each product upgrade. But one question remains: where are the long-lasting batteries?
A collaboration of researchers recently made a discovery that might hold the answer to that question: a new liquid electrolyte material that conducts fluoride in fluoride-based rechargeable batteries, which pack a major energy punch. As part of the project, a team led by the California Institute of Technology’s (Caltech’s) Thomas Miller used the 27-petaflop Cray XK7 Titan supercomputer at the Oak Ridge Leadership Computing Facility (OLCF) to understand and refine the electrolyte’s properties and confirm its unprecedented ability to conduct fluoride ions and retain chemical stability at room temperature, making the breakthrough material the first of its kind in the battery world.
“We’ve taken an exciting step in the right direction,” Miller said. “We have helped develop electrolytes that are sufficiently stable to make practical fluoride ion batteries a more achievable goal.”
Fluoride batteries—which employ the same element that’s added to toothpaste and tap water to prevent tooth decay—have long been studied for their potential to hold more charge than lithium batteries. However, these batteries have previously only worked with solid electrolytes that require high temperatures to operate, rendering them impractical for everyday applications.
“The problem with solid electrolytes is that the rigidity of the solid prevents the needed motion of the fluoride ions at a useful rate,” Miller said.
Victoria Davis, an intern working under Jet Propulsion Laboratory (JPL) chemist Simon Jones, first proposed the new material—and it worked. Simulations performed by Miller’s team then revealed the reason for the success of the electrolyte and guided its further improvement on the basis of the simulation predictions.
“This liquid electrolyte allows for much more facile motion of the fluoride ions, even at room temperature, and its interactions help stabilize the fluoride,” Miller said.
The study, published last week in Science, included chemists from Caltech, JPL, the Honda Research Institute, and Lawrence Berkeley National Laboratory (Berkeley Lab).
The finding is crucial because the more energy-dense fluoride batteries could hold up to 8 times more charge than lithium batteries. The discovery gives researchers a map for the mechanisms involved in stabilizing fluoride batteries and could aid in the development of new kinds of batteries with applications in cars, cell phones, or other electronic devices.
Turning down the heat
The electrolyte in a battery system plays an important role in its operation. The electrolyte—whether solid or liquid—promotes the movement of ions from one end of a battery to the other, while the electrons move through a circuit out of the battery to power an electronic device on the way to the cathode, the positive terminal.
Lithium-based batteries work by shuttling positively charged lithium ions from the negative terminal of a battery (the anode) to the cathode and back, like grains of sand in an hourglass, without undergoing chemical reactions. Fluoride-based batteries, though, operate differently.
In the team’s fluoride battery, chemical reactions occur at the electrodes, where negatively charged fluorine anions form and break bonds to convert chemical energy to electrical energy. Fluorine anions can store a great deal of energy per ion, making them an attractive candidate for rechargeable batteries.
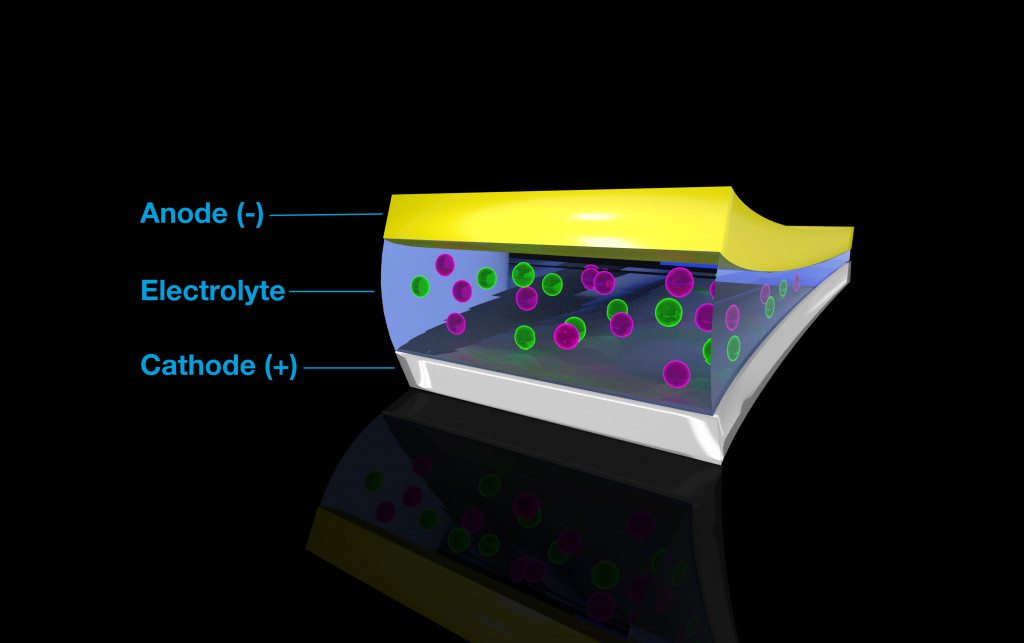
An illustration of a battery. In order for batteries to generate electricity, charged atoms, called ions (pink and green), travel between a negative node (anode) and a positive node (cathode) with the help of a liquid electrolyte solution. Image Credit: Brett Savoie, Purdue University
“The ion you use in a battery determines how much energy you can potentially store per ion in each electrode,” said Brett Savoie, an assistant professor at Purdue University who worked on this project as a postdoctoral fellow at Caltech. “Fluoride-based batteries are attractive because fluorine is the most electronegative element, so they can operate at high voltages and make for successful high-density batteries.”
The problem, though, has been finding a suitable room-temperature electrolyte—the conductor material that plays a crucial role in shuttling the ions around inside a battery.
Solid electrolytes can conduct fluoride safely and are known to be more stable than liquid electrolytes, but they are costly and require high temperatures to operate. For fluoride to become a viable option for rechargeable battery technology, it would require a stable liquid electrolyte. A collaborative team featuring scientists from JPL, Caltech, Honda, and Berkeley Lab decided to try a new liquid electrolyte called bis(2,2,2-trifluoroethyl)ether (BTFE). Remarkably, the new solvent worked and made the fluoride ions stable enough to shuttle from the anode to the cathode—which enables the battery to drive electrical current. But the team didn’t quite know why.
Using the OLCF’s Titan supercomputer, Miller’s team used the Large-Scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) code to conduct molecular dynamics simulations on Titan under an Innovative and Novel Computational Impact on Theory and Experiment, or INCITE, allocation to look at BTFE’s reactions with fluoride. The OLCF is a US Department of Energy (DOE) Office of Science User Facility located at DOE’s Oak Ridge National Laboratory.
The team discovered that positively charged regions in BTFE were strongly interacting with the negatively charged fluoride to dissolve it.
“BTFE is one of the few organic solvents that we know of that can dissolve fluoride salts at appreciable levels without decomposing,” Savoie said. “The key to this was that it could also stabilize the fluoride at room temperature.”
Fluoride batteries for the future
The team used LAMMPS and the OLCF’s Titan to explain the chemistry of BTFE and then proposed other molecules that would behave similarly. The group simulated other molecules as additives to BTFE and found they further improved the electrolyte’s performance.
“Once you understand, at a molecular level, why something is working, you can design improvements on that,” Miller said. “So we helped provide the fundamental understanding of why it was working and then offered design improvements based on that.”
The team performed simulations over a 6-month period to capture a few microseconds of battery interactions, including many different solvents and positively charged ions—which are required to balance out the negatively charged fluoride ions in the electrolyte. Among the other solvents tested were BPFE, a chemically similar but less successful solvent, and diglyme, a solvent that could potentially expand the voltage window of BTFE and make it even more stable.
The Caltech/JPL/Honda team is now performing follow-up studies and developing advances to optimize the electrolyte.
“We will need to do more work before this can become a viable technology for widespread deployment,” Miller said. “But we know that fluoride ion batteries certainly hold promise as a future battery technology.”
Related Publication: Davis, V. K., C. M. Bates, K. Omichi, B. M. Savoie, N. Momčilović, Q. Xu, W. J. Wolf, M. A. Webb, K. J. Billings, N. H. Chou, S. Alayoglu, R. K. McKenney, I. M. Darolles, N. G. Nair, A. Hightower, D. Rosenberg, M. Ahmed, C. J. Brooks, T. F. Miller III, R. H. Grubbs, and S. C. Jones, “Room Temperature Cycling of Metal Fluoride Electrodes: Liquid Electrolytes for High-Energy Fluoride Ion Cells.” Science 362, no. 6419 (2018): 1144–1148, doi: 10.1126/science.aat7070.
ORNL is managed by UT-Battelle for the Department of Energy’s Office of Science, the single largest supporter of basic research in the physical sciences in the United States. DOE’s Office of Science is working to address some of the most pressing challenges of our time. For more information, please visit https://science.energy.gov.