Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.
Before determining which underlying properties of solid materials are crucial for improving these applications, researchers must better understand the factors that control ionic conduction. To pursue this knowledge, a multidisciplinary team from the US Department of Energy’s (DOE’s) Oak Ridge National Laboratory (ORNL) developed a computational framework to process and analyze large datasets of ion-conducting solids.
Using a dataset containing over 80 different compositions of materials called perovskites, the researchers focused primarily on identifying and optimizing those with promising proton conduction capabilities. These novel materials could enable the production of more reliable and efficient proton-conducting solid oxide fuel cells—energy storage devices that convert chemicals into electricity for practical uses such as powering vehicles.
Results from this work are published in The Journal of Physical Chemistry and Chemistry of Materials, and members of the team also presented their findings at the Materials Research Society’s Fall Meeting in 2018.
“We are looking for better ionic conducting materials because, in any solid electrolyte used for fuel cells or batteries, the faster the ions move, the more efficiently the device will operate,” said principal investigator Panchapakesan Ganesh, an R&D staff member at ORNL’s Center for Nanophase Materials Sciences (CNMS). “We now have an understanding that will help us come up with new design principles for developing such materials.”
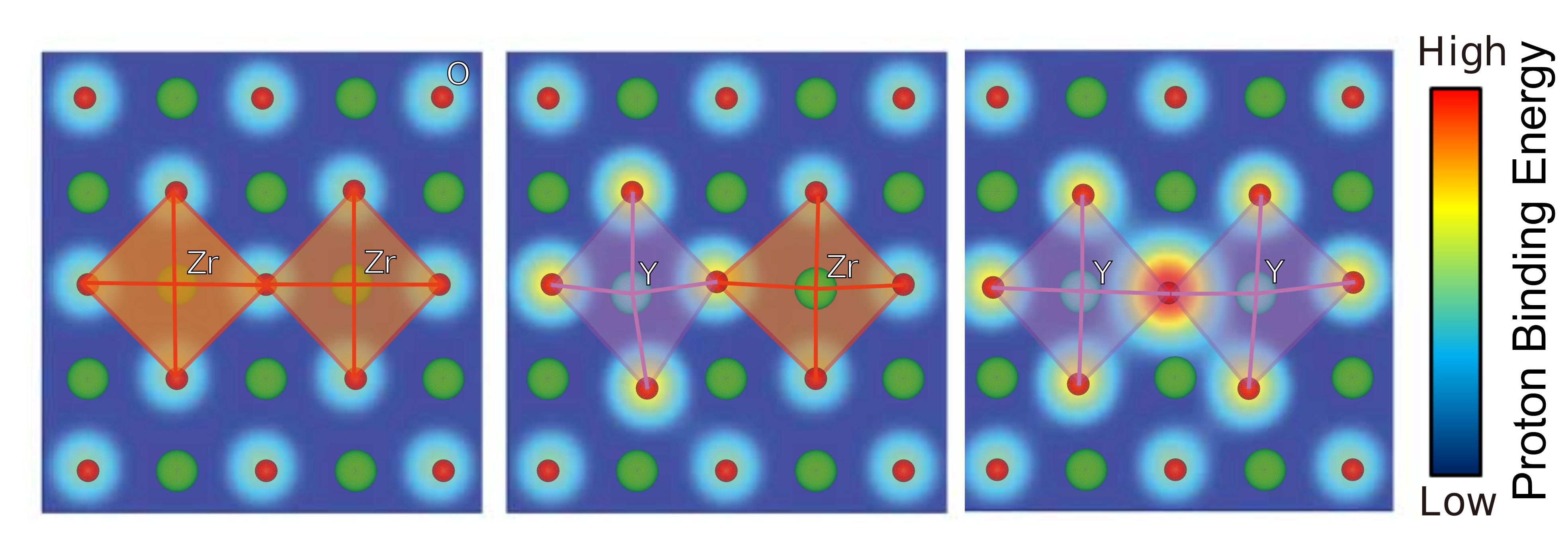
The team studied materials including one of the fastest known proton conductors, an altered version of the compound barium zirconate (BaZrO3) formed by replacing zirconium (Zr) with yttrium (Y), an element that reduces the overall charge of the compound to facilitate the addition of protons. Elements that exhibit this behavior are called acceptor dopants, and the material in question is often referred to as yttrium-doped BaZrO3, or Y-BZO.
Systematically screening so many candidates from the perovskite dataset in a short time would not have been possible without the computing power of Titan, a Cray XK7 supercomputer housed at the Oak Ridge Leadership Computing Facility (OLCF). Using multiple codes and a computational tool called wraprun, OLCF staff members helped the team develop an automated workflow optimized for Titan’s architecture.
“We worked closely with OLCF staff to build a highly scalable workflow that allowed us to use thousands of cores simultaneously on Titan,” Ganesh said.
These simulations revealed that correlations between lattice distortions and proton binding energy—the amount of energy required to separate a proton from a perovskite material—can make protons heavier and slower, inhibiting optimal proton conduction. This revelation could help the researchers identify existing materials and develop new ones able to compete with Y-BZO.
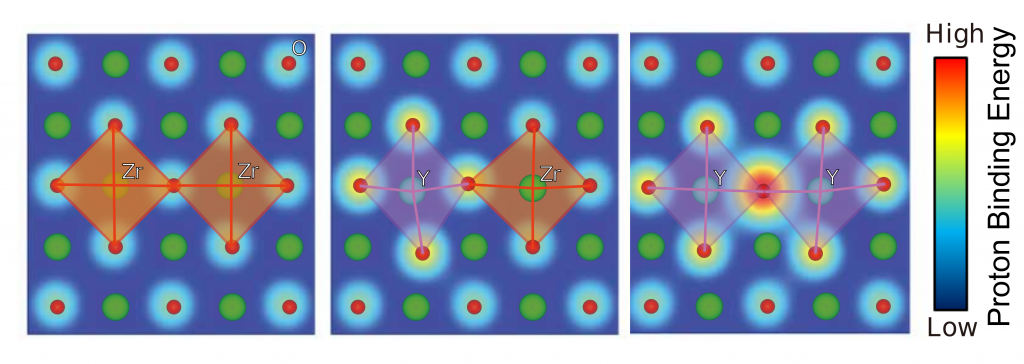
The illustrations show how the correlation between lattice distortion and proton binding energy in a material affects proton conduction in different environments. Mitigating this interaction could help researchers improve the ionic conductivity of solid materials.
“We realized that the coupling of mobile ions with distortions in the crystal lattice is one of the most important ingredients for ionic conduction,” Ganesh said. “Understanding this connection means we can selectively design solid materials with improved ionic conductivity.”
In addition to the practical benefits these results could have for energy applications, the team’s newfound knowledge provides fundamental insights into scientific concepts.
“During this process of understanding what limits proton conduction in existing materials, we hope to also discover some new physics,” Ganesh said. “It’s all related to underlying atomistic mechanisms.”
To validate the computational results, members of the team conducted a series of complementary experiments that employed pulsed laser deposition, scanning transmission electron microscopy, time-resolved Kelvin probe force microscopy, and atom probe tomography techniques at CNMS, as well as neutron scattering at the Spallation Neutron Source (SNS). CNMS, SNS, and the OLCF are all DOE Office of Science User Facilities located at ORNL.
The researchers plan to expand their efforts beyond protons and perovskites to investigate the behavior of mobile ions in other categories of materials. Future findings could enhance the performance of other types of fuel cells, as well as lithium-ion batteries.
“The computing framework developed to study doped perovskites can be applied to other types of crystalline inorganic solids, and the availability of such large defect datasets allows us to leverage ORNL’s expertise in advanced artificial intelligence techniques to accelerate material discovery,” Ganesh said.
Funding for this research came from the Laboratory Directed Research and Development Program at ORNL.
Related publications:
Janakiraman Balachandran, Lianshan Lin, Jonathan S. Anchell, Craig A. Bridges, and P. Ganesh, “Defect Genome of Cubic Perovskites for Fuel Cell Applications.” The Journal of Physical Chemistry 121, no. 48 (2017): 26637–26647, doi:10.1021/acs.jpcc.7b08716.
Jilai Ding, et al., “The Influence of Local Distortions on Proton Mobility in Acceptor Doped Perovskites.” Chemistry of Materials 30, no. 15 (2018): 4919–4925, doi:10.1021/acs.chemmater.8b00502.
UT-Battelle LLC manages Oak Ridge National Laboratory for DOE’s Office of Science, the single largest supporter of basic research in the physical sciences in the United States. DOE’s Office of Science is working to address some of the most pressing challenges of our time. For more information, please visit https://science.energy.gov.