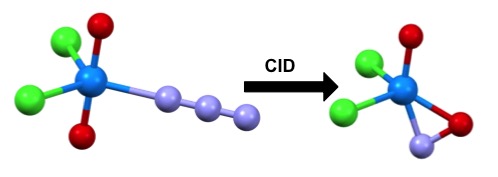
Interpreting the results of collision induced dissociation (CID) experiments, simulations on Titan predict the formation of an unusually bonded uranium-nitrosyl molecule. Credit: J. Am. Chem. Society. DOI: 10.1021/jacs.5b02420
Multi-institution team works on chemical methods to clean up nuclear waste
Radioactive materials have long been a part of American history—from the Manhattan Project to the development of nuclear power. The materials central to these innovations are actinides, or elements 89–103 on the periodic table that release large amounts of energy when atoms are split.
That same energy also makes actinides highly radioactive. Scientists doing early research with these substances in the 1940s and 50s did not fully understand the risks of long-term handling and storage for the resulting waste products. Locations such as the Department of Energy’s (DOE’s) Hanford and Savannah River Sites still have radioactive materials from the Manhattan Project in storage awaiting processing and final disposition.
Often stored in large holding containers, these volatile substances mix with one another over time. One of the major challenges for decontaminating these sites lies in separating radioactive compounds from their more inert counterparts for safe removal and storage.
To this end, a multi-institution team has been using computing resources at DOE’s Oak Ridge National Laboratory (ORNL) to understand actinide chemistry at the molecular level in hopes of designing methods to clean up contamination and safely store spent nuclear fuel.
“We put this group together because we were already collaborating on scientific papers,” said professor David Dixon of The University of Alabama. “Rather than just saying that we were going to do one reaction and needed computing time, we’re using a team approach to try and get a broad, fundamental understanding of actinide science.”
Dixon serves as the principal investigator on the project, which includes collaborators from Lawrence Berkeley National Laboratory, Washington State University, The State University of New York at Buffalo, the University of Minnesota, and Rice University.
The team is using the Titan supercomputer at the Oak Ridge Leadership Computing Facility—a DOE Office of Science User Facility located at ORNL—to work on a variety of research goals related to understanding the chemical reactions taking place with actinides.
Supercomputing plays an essential role in understanding these elements. Because of their high radioactivity, actinides are difficult to handle, and those experiments that can be performed are expensive. “These elements are really radioactive, and in many cases, there is not much of it around,” Dixon said. “The radioactivity part makes it very difficult to handle in a lab. We want to try and get as much information as we can about their properties before people do specific lab experiments to test out ideas and design new systems to help remediate contamination.”
Attacking actinide bonds
The Dixon team gained access to Titan through DOE’s Innovative and Novel Computational Impact on Theory and Experiment (INCITE) program. Within the larger allocation of computing time, the team is divided into several smaller groups looking at specific problems related to actinide chemistry. The team’s research was also funded by DOE’s Basic Energy Sciences (BES) program.
After spent nuclear fuel begins to dissolve within a storage tank, actinides still exist as ions in an aqueous mixture. To clean up this mixture, researchers must separate these ions into groups based on how they affect the remediation process and by the radioactive hazards they present.
Currently, state-of-the-art methods revolve around a multistage process where researchers form a metal-ligand complex with an actinide ion, which then helps the ion migrate across the boundary between oil and water.
Washington State University professor Aurora Clark leads a group that studies how solutions of actinides can be separated, primarily focusing on liquid–liquid interfaces.
“One of the most critical components of actinide separation is the interface between water or an aqueous solvent system and an organic solvent (such as oil), and often these mixtures are incredibly complicated,” Clark said. “We’re doing simulations to understand the essential physics of what’s going on at these liquid–liquid interfaces. We’re trying to understand how the structure and dynamics at the oil–water interface can influence permeability of different compounds across these interfaces and can influence the ability of an actinide-containing molecule to migrate across the interface.
Essentially, much of the storage for radioactive waste needs to be cleaned of actinides. Clark’s group runs large-scale molecular dynamics (MD) simulations to understand this process with a level of detail unattainable through experiment.
The team uses Titan to design a solution containing thousands to tens of thousands of individual atoms. During the simulation, the team charts the particles’ interactions as the solution moves to a state of equilibrium—or where a reaction and any counter-reaction proceed at an equal rate—and then analyzes the data from these interactions.
Clark’s group uses some novel approaches for data analysis. One of the team’s achievements was creating intermolecular network theory, which allows the team to focus less on the positions of each individual particle in the simulation (like many MD simulations) and more on how the interactions of various particles dictate the physical properties of these radioactive mixtures.
Intermolecular network theory gets the team to take its data and analyze a “network” of the various chemical interactions. Researchers then analyze that network much like a network analyst would observe internet connectivity or monitor a network of computers—in fact, the team uses a modified version of Google’s PageRank algorithm, which is instrumental in ranking the importance and relevance of web content, to help understand the immediate organization of solvents around ions.
Through its suite of computational tools and access to Titan in the last year, the Clark group was able to observe how the molecule tributyl phosphate changes its conformation when at the interface of water and hexane. As the molecule changes, a massive reorganization at the interface makes the transfer of an actinide across the interface more permeable.
In addition to working on liquid–liquid interfaces, Clark’s group is also starting to simulate liquid–solid interactions, with a specific focus on metal organic frameworks—compounds that create nanoscale pores that attract and bind to actinides. As supercomputing power continues to increase, Clark hopes that metal organic frameworks may become the gold standard for scrubbing actinides from an environment. “The strategy [of employing metal organic frameworks] compliments our other research and provides a way to both separate and potentially store radioactive material in a single step,” Clark said.
Clark’s group is generating large amounts of data in these simulations and has been working with OLCF support staff to develop methods for in situ data analysis techniques that would allow the team to drastically reduce the amount of data it would need to transport out of the OLCF.
Experimental emphasis
Other researchers on the Dixon team, such as Lawrence Berkeley National Laboratory’s (LBNL’s) Bert de Jong, are using their time on Titan and the OLCF’s developmental and analysis cluster Eos to collaborate closely with experimentalists. De Jong’s group works with teams at both LBNL and the University of Utah to understand how to change and control an element’s oxidation state—or how many electrons an atom can shed or add to change its charge.
By altering the behavior of elements like uranium—or its actinide relatives, thorium, neptunium, and plutonium—incorporating them in molecules and forming chemical bonds, researchers hope to extract radioactive elements from the environment or waste storage tanks and keep them in a more innocuous form, or to separate them for reuse as nuclear fuel.
De Jong’s group benefitted from Titan’s hybrid architecture. “For our thorium work, we had to do very high-accuracy simulations, and for those, we have to do coupled cluster calculations,” de Jong said. “The NWChem code that we use is GPU enabled, so that allows us to do these kinds of calculations with the fastest time-to-solution possible.”
Throughout the group’s work on Titan and Eos, simulations have served as both the basis for driving experimental research and verification of certain experimental hypotheses. “I believe that computing is both predictive and interpretive,” de Jong said. “Experiments often can only tackle aspects of the problem, but computing can help broaden insights and trends that can confirm or change interpretations and provide more additional information very quickly and safely.”
De Jong pointed to research on chemical reactions involving ligands and proteins—molecules that bind to radioactive elements—where Titan simulations suggested that certain reactions would shed water molecules while reducing uranium. This phenomenon was subsequently observed experimentally in the Netherlands. Simulations on Titan also predicted the formation of an unusually bonded uranium-nitrosyl molecule, work that was published in the Journal of the American Chemical Society.
“Simulations on elements on the bottom of the periodic table, such as uranium and plutonium, are a challenge, and we are using accurate experimental data to verify that our models and methods are accurate,” de Jong said. The verification of experiments performed at the University of Utah, studying thorium reactions in a gas phase, lays the foundation for complex simulations of uranium or heavier, more volatile elements that are dangerous to work with in a laboratory.
As the OLCF moves toward its next-generation supercomputer, Summit, the Dixon team plans to focus on optimizing its various codes for taking full advantage of GPUs and other computing methods. The team wants to continue expanding its work on metal organic frameworks and incorporate quantum Monte Carlo methods—a computational technique that uses statistical data and random numbers to accurately plot electrons in a simulation.
Related Publications:
R. Cox, P. B. Armentrout, and W. de Jong, “Reactions of Th + H2, D2, and HD Studied by Guided Ion Beam Tandem Mass Spectrometry and Quantum Chemical Calculations.” The Journal of Physical Chemistry B (2015), doi:10.1021/acs.jpcb.5b08008.
Y. Ghadar, P. Parmar, A. Samuels, and A. Clark, “Solutes at the Liquid:Liquid Phase Boundary—Solubility and Solvent Conformational Response Alter Interfacial Microsolvation.” The Journal of Chemical Physics 142 (2015), doi:10.1063/1.4914142.
Y, Gong, W. de Jong, and J. Gibson, “Gas Phase Uranyl Activation: Formation of a Uranium Nitrosyl Complex from Uranyl Azide.” Journal of the American Chemical Society (2015), doi:10.1021/jacs.5b02420.
Oak Ridge National Laboratory is supported by the US Department of Energy’s Office of Science. The single largest supporter of basic research in the physical sciences in the United States, the Office of Science is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.