Neuromorphic devices — which emulate the decision-making processes of the human brain — show great promise for solving pressing scientific problems, but building physical systems to realize this potential presents researchers with a significant challenge. An international team has gained additional insights into a material compound called vanadium oxide, or VO2, that might be the missing ingredient needed to complete a reliable neuromorphic recipe.
VO2, which belongs to a class of materials known as correlated solid oxides, must undergo a reversible transformation from its insulating form to a metallic form to become a practical candidate for this purpose. And although atomic imperfections called point defects are capable of optimizing materials for technological applications, the specific effects of such alterations, which are needed to enhance the compound’s functional qualities, were previously unknown.
Motivated by this knowledge gap, researchers from the Department of Energy’s Oak Ridge and Argonne national laboratories, Tampere University and the University of Hamburg applied complementary many-body theoretical methods at multiple computing facilities to obtain new insights into VO2’s interactions with different types of point defects. The researchers created the most complete picture of this complex compound’s transformation to date and their findings are published in Physical Review B.
There are at least two varieties of point defects: a vacancy, in which an atom is removed from a material’s crystal structure, and a substitution, in which one atom is removed from the structure and replaced with a different atom. By adjusting vacancies and substitutions in a material through a process called doping, researchers can enable previously impossible applications, from improving energy storage capabilities to streamlining neuromorphic computing research.
A metal of mettle
Having uncovered an unprecedented view into VO2’s fundamental structure and behavior, the team answered a long-standing physics question that asks whether electron correlations or intrinsic structural instabilities are the underlying mechanisms responsible for a phenomenon called the metal-insulator transition, or MIT.
Normally, VO2 exists as a metal at high temperatures and as an insulator at low temperatures, switching between these two states through MIT in accordance with its surroundings. The metal is classified as a “bad metal” and is characterized by unusually high resistivity due to strong electron-electron correlations, whereas the insulator has a distorted crystal structure.
The team discovered that introducing oxygen vacancies into the metal suppressed the natural MIT process and allowed VO2 to remain in the metallic state, even at low temperatures. Observing how vacancies suppressed the insulating state helped the researchers determine that electron correlations, not structural instabilities, are essential for triggering the structural distortions that eventually lead to the MIT transition.
Because a metallic material will conduct electrons but an insulator will not, MIT essentially acts as a switch. Fine-tuning the control of MIT in correlated solids by injecting vacancies at any time means that researchers can make VO2 a prime candidate for constructing novel neuromorphic systems.
“We identified a single, fundamental knob that allows us to control complex coupled phase transitions in correlated solids,” said lead author Panchapakesan Ganesh, a researcher at ORNL’s Center for Nanophase Materials Sciences, or CNMS, a DOE Office of Science user facility. “This ability could be relevant for engineering technologically appropriate material systems for next-generation neuromorphic devices.”
Combining computing methods
The team also studied the differences between VO2’s properties in the ground state — the energy level that the compound exhibits in a neutral environment — and the excited state — the increased energy level that the compound exhibits when its electrons are excited through interactions with various external particles, such as photons. This study marks one of the first times any team has successfully characterized both the ground and excited state properties within a single solid oxide, an accomplishment made possible by the researchers’ computationally intensive measurement methods.
Using resources at the National Energy Research Scientific Computing Center, a DOE Office of Science user facility at DOE’s Lawrence Berkeley National Laboratory, the researchers began by identifying the positions of atoms in VO2 with the density functional theory, or DFT, method.
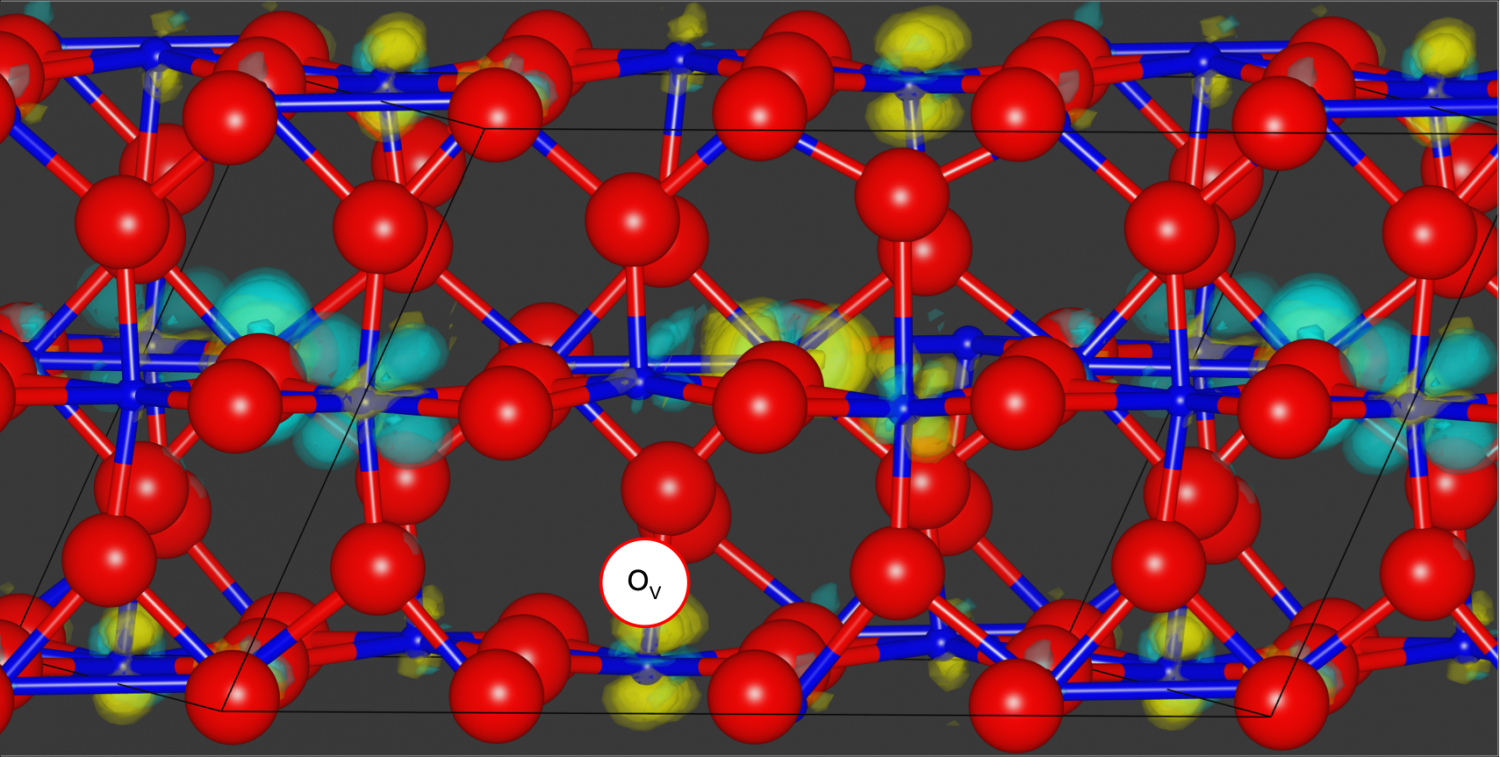
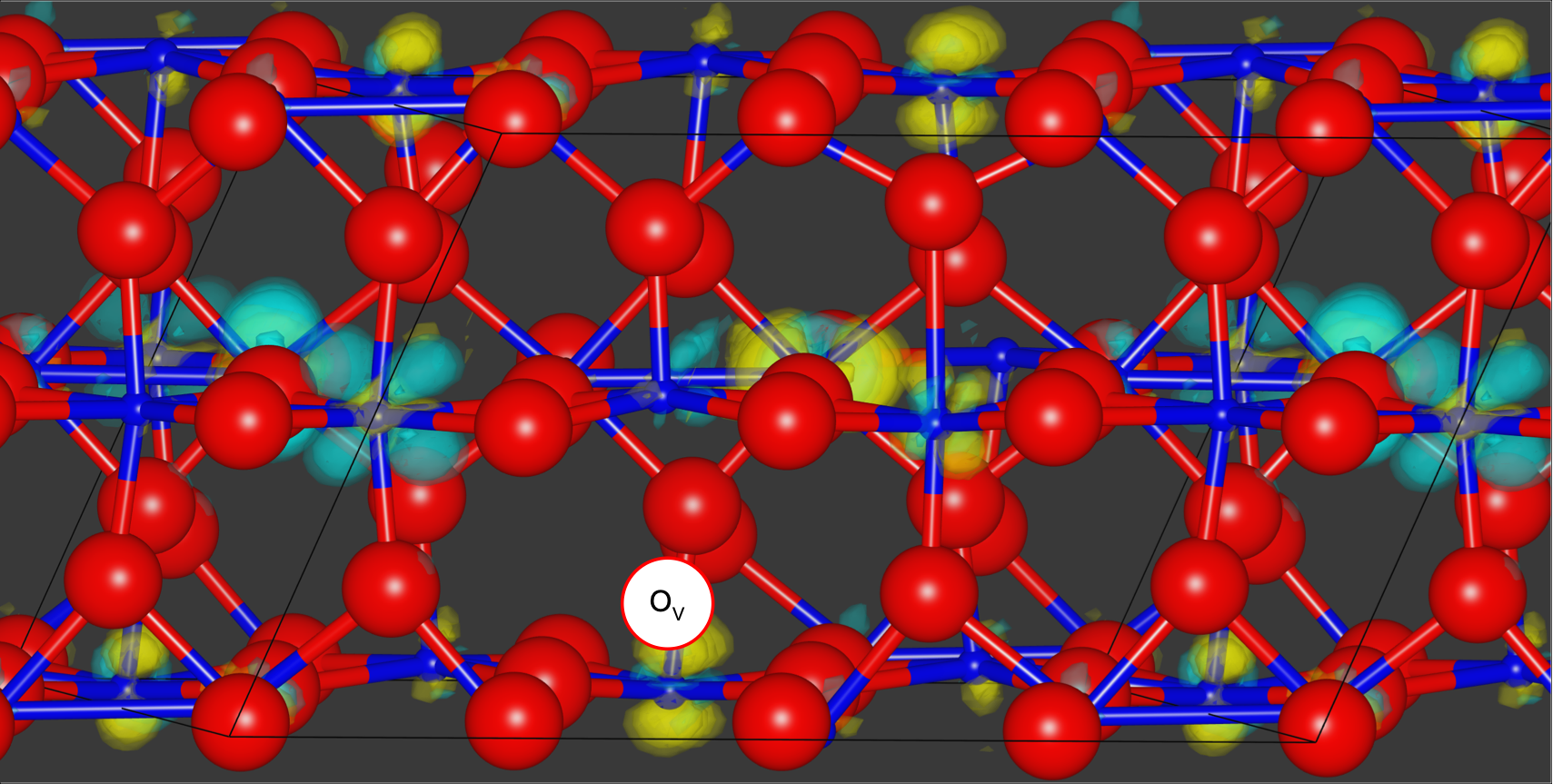
Using quantum Monte Carlo methods, the researchers simulated bulk VO2. Yellow and turquoise represent changes in electron density between the excited and ground states of a compound composed of oxygen, in red, and vanadium, in blue, which allowed them to evaluate how an oxygen vacancy, in white, can alter the compound’s properties. Credit: Panchapakesan Ganesh/ORNL, U.S. Dept. of Energy
Based on those results, they used the now-decommissioned Titan supercomputer to complete a diffusion Monte Carlo, or DMC, calculation. This accurate, many-body method designed to analyze solid materials revealed the compound’s ground state properties, such as the amount of energy needed to support the addition of a vacancy and the distribution of an electric charge. Titan was located at the Oak Ridge Leadership Computing Facility, a DOE Office of Science user facility at ORNL.
Finally, the team used complementary methods, namely the combination of DFT and the dynamical mean field theory, known collectively as DFT + DMFT, to evaluate VO2’s elusive excited state properties, such as the distribution of all electronic states that could be populated by interactions with photons or other particles. The researchers completed this portion of the project on a computing cluster provided by the Julich Supercomputing Centre. Benchmarking DFT and DFT + DMFT ground state approximations against the precise results provided by DMC secured their confidence in the validity of the excited state calculations.
“Comparing results from these advanced methods proved that they were internally consistent with each other and that the physics we observed was accurate,” Ganesh said. “Additionally, comparing the simulation results to output from practical experiments demonstrated that the quantities we calculated were measurable and consistent with real data.”
Other oxides
In a related study, an overlapping team from ORNL, Argonne, the University of Illinois at Chicago, the University of Illinois at Urbana-Champaign, Northwestern University and the Ulsan National Institute of Science and Technology used the same approach to better understand another solid oxide called nickel oxide, or NiO. Findings from this follow-up research are published in a separate Physical Review B paper.
The researchers evaluated how doping NiO with n-type defects — which add more electrons to the compound — and p-type defects — which create holes in the compound — can be harnessed to control MIT and amplify useful properties such as magnetoresistance and superconductivity with the goal of optimizing NiO for electronics applications.
They used resources at CNMS, the Argonne Center for Nanoscale Materials and the Argonne Leadership Computing Facility, a DOE Office of Science user facility at Argonne, through the same Innovative and Novel Computational Impact on Theory and Experiment program allocation that supported the VO2 research at the OLCF.
“Because we successfully completed an in-depth study of vacancy defects in the strongly correlated compound VO2, we followed those simulations with an in-depth study of substitutional defects in the strongly correlated compound NiO,” Ganesh said. “A crucial difference is that all the NiO experiments were performed by our team, which was a major undertaking. National laboratories such as ORNL are the perfect places for tying discoveries in fundamental science and synthesis of materials to the physics of devices to create novel computing architectures.”
Now, researchers from both teams are studying spin orbit coupling, an intangible interaction that occurs between particles in quantum materials. They anticipate that the methods used to control physical defects could also be extrapolated to harness the power of this interaction, which could help optimize materials used in the development of quantum computers.
Research conducted at ORNL and Argonne was supported by the DOE Office of Science. Portions of this work were supported by the National Research Foundation of Korea.
UT-Battelle manages Oak Ridge National Laboratory for DOE’s Office of Science, the single largest supporter of basic research in the physical sciences in the United States. DOE’s Office of Science is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.